Abstract
Introduction: Limited data exist regarding patient-reported outcomes (PROs) of chimeric antigen receptor (CAR) T-cell therapy, with no published longitudinal studies to our knowledge reporting on patients treated as standard of care. The goal of the current study was to report on real-world changes in PROs (i.e., patient-reported symptoms and quality of life) in the first year after treatment with axicabtagene ciloleucel (axi-cel).
Methods: Patients scheduled to receive axi-cel outside the context of a clinical trial were recruited between March 2020 and June 2022. They completed a baseline questionnaire at recruitment prior to conditioning chemotherapy before axi-cel. Patients completed subsequent questionnaires at 14, 30, 60, 90, 180 and 360 days post-infusion of axi-cel. PROs were assessed using the EORTC QLQ-C30. Mixed models evaluated change in EORTC global quality of life and subscales using all available data. Meaningful change was defined as a difference of 10 points, consistent with previous studies.1
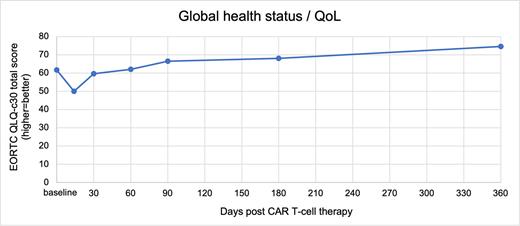
Results: A total of 50 patients participated [mean age 62 (SD=13); 64% male; 72% diffuse large B cell lymphoma; 14% transformed follicular lymphoma, 14% follicular lymphoma]. The majority of patients (64%) would have been eligible at apheresis for the ZUMA-1 pivotal axi-cel trial; those who were ineligible typically had follicular lymphoma, poor performance status, and/or comorbidity. Regarding PROs, despite transient worsening at day 14, statistically-significant improvements in global quality of life (Panel 1), physical functioning, role functioning, social functioning, fatigue, insomnia, appetite loss, and constipation were observed over the course of the year (p values <0.05). Of these, clinically-significant improvements were observed at day 360 relative to baseline in global quality of life, role functioning, fatigue, insomnia, appetite loss, and constipation. Pain and financial concerns demonstrated statistically and clinically meaningful improvements to day 180, then worsened to baseline (i.e., pre-conditioning) levels or slightly below at day 360 (p values <0.05).
Conclusions: Real-world data suggest that axi-cel is associated with transient worsening of quality of life and symptoms at day 14, with statistically and clinically significant improvements thereafter in overall quality of life and several functional and symptom domains. These data are generally consistent with PROs reported from clinical trials of CAR T-cell therapy. However, these findings extend previous research by reporting on patients' perspectives on CAR T-cell therapy received as standard of care in the real-world setting.
1Osoba, D., Rodrigues, G., Myles, J., Zee, B., & Pater, J. (1998). Interpreting the significance of changes in health-related quality-of-life scores. Journal of Clinical Oncology, 16(1), 139-144.
Panel 1. Change in overall quality of life in the year following receipt of axicabtagene ciloleucel as part of standard of care.
Disclosures
Jim:Kite Pharma: Research Funding. Thornton Snider:Kite Pharma: Current Employment, Current equity holder in publicly-traded company. West Wade:Kite Pharma: Consultancy; Abbvie: Consultancy; Johnson & Johnson: Consultancy. Fu:Kite Pharma: Current Employment, Current equity holder in publicly-traded company; Amgen: Current equity holder in publicly-traded company; Cellares: Patents & Royalties. Chavez:Epizyme: Honoraria, Speakers Bureau; Adicet: Consultancy; Kite Pharma: Consultancy; GenMab: Consultancy; MorphoSys/Incyte: Speakers Bureau; Astrazeneca: Research Funding, Speakers Bureau; ADC Therapeutics: Research Funding; Janssen: Research Funding; TG Therapeutics: Honoraria; Beigene: Honoraria; Merck: Research Funding; Abbvie: Consultancy. Jain:BMS: Consultancy; MyeloidTx: Consultancy; Incyte: Research Funding; Novartis: Consultancy; Kite Pharma: Consultancy, Research Funding. Lazaryan:AmWel: Current equity holder in publicly-traded company; Teladoc: Current equity holder in publicly-traded company; Humanigen: Consultancy; AvroBio: Consultancy; Sanofi: Consultancy. Shah:PeproMene Bio: Other: Steering committee; Servier: Other: grants and investigator-initiated trials; Autolus: Consultancy; Century Therapeutics: Consultancy; Adaptive: Consultancy; Pharmacyclics: Consultancy; Beigene: Consultancy; Acrotech: Consultancy; Jazz: Consultancy, Other: grants and investigator-initiated trials; Precision Biosciences: Consultancy; Kite/Gilead: Consultancy, Other: grants and investigator-initiated trials; BMS/Celgene/Juno: Consultancy; Novartis: Consultancy; Pfizer: Consultancy; Amgen: Consultancy. Locke:Leukemia and Lymphoma Society: Research Funding; ), National Cancer Institute: Research Funding; CERo Therapeutics: Research Funding; CAREducation: Other: Education or editorial activity; BioPharm Communications: Other: Education or editorial activity; Takeda: Consultancy; Sana: Consultancy; Aptitude Health: Other: Education or editorial activity; Society for Immunotherapy of Cancer: Other: Education or editorial activity; Imedex: Other: Education or editorial activity; ASH: Other: Education or editorial activity; BMS: Research Funding; Daiichi Sankyo: Consultancy; Clinical Care Options Oncology: Other: Education or editorial activity; A2: Consultancy; Celgene: Consultancy; Other: Patents & Royalties: patents, royalties, other intellectual property from several patents held by the institution in my name (unlicensed) in the field of cellular immunotherapy.; Wugen: Consultancy; Umoja: Consultancy; Novartis: Consultancy, Research Funding; Legend Biotech: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Janssen: Consultancy; Iovance: Consultancy; GammaDelta Therapeutics: Consultancy; Emerging Therapy Solutions Gerson Lehrman Group: Consultancy; EcoR1: Consultancy; Cowen: Consultancy; Calibr: Consultancy; Cellular Biomedicine Group: Consultancy; Bristol Myers Squibb/Celgene: Consultancy; Bluebird Bio: Consultancy, Research Funding; Allogene: Consultancy, Research Funding; Amgen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal